Supramoleculartrap for catching polyamines in cells as an anti-tumor strategy
Junyi Chen1,2, Hanzhi Ni2,3, Zhao Meng2,Jing Wang2, Xiayang Huang3, Yansheng Dong2,Chao Sun2,Yadan Zhang2, Lei Cui3, Jian Li3,Xueshun Jia1,3, Qingbin Meng2, Chunju Li1,3,4(李春举)
1Collegeof Environmental and Chemical Engineering, Shanghai University, Shanghai200444, P. R. China
2StateKey Laboratory of Toxicology and Medical Countermeasures, Beijing Institute ofPharmacology and Toxicology, Beijing 100850, P. R. China
3Departmentof Chemistry, Center for Supramolecular Chemistry and Catalysis, ShanghaiUniversity, Shanghai 200444, P. R. China
4KeyLaboratory of Inorganic-Organic Hybrid Functional Material Chemistry, Ministryof Education, Tianjin Key Laboratory of Structure and Performance forFunctional Molecules, College of Chemistry, Tianjin Normal University, Tianjin 300387,P. R. China
Nat.Commun.2019, 10, 3546.
Abstract: Polyamines are essential for the growth ofeukaryotic cells and can be dysregulated in tumors. Here we describe a strategyto deplete polyamines through host–guest encapsulation using apeptide-pillar[5]arene conjugate (P1P5A, P1 = RGDSK(N3)EEEE)as a supramolecular trap. The RGD in the peptide sequence allows the moleculeto bind to integrin αvβ3-overexpressing tumor cells. Thenegative charged glutamic acid residues enhance the inclusion affinitiesbetween the pillar[5]arene and cationic polyamines via electrostaticinteractions and facilitate the solubility of the conjugate in aqueous media.The trap P1P5A efficiently encapsulates polyamines with association constantsof 105–106 M−1. We show that P1P5A has a wide spectrum ofantitumor activities, and induces apoptosis via affecting the polyaminebiosynthetic pathway. Experiments in vivo show that P1P5A effectively inhibitsthe growth of breast adenocarcinoma xenografts in female nude mice. This workreveals an approach for suppressing tumor growth by using supramolecularmacrocycles to trap polyamines in tumor cells.
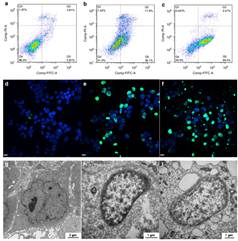
链接:https://doi.org/10.1038/s41467-019-11553-7
 目的地搜索
目的地搜索